
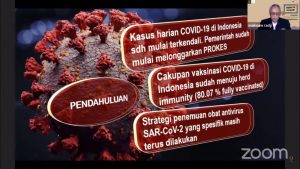
The Student Executive Board, Faculty of Pharmacy, Universitas Indonesia (FPUI), held a public discussion to review a series of research and developments that have been carried out by medical personnel as a curative strategy for Covid-19 by presenting the Director of Production Supervision of Drugs, Narcotics, Psychotropics, and Precursors of BPOM, apt. Dra. Togi Junice Hutadjulu, MHA. “Currently, there are four types of drugs that have obtained EUA (Emergency Use Authorization) permits or as Covid-19 therapeutic drugs in Indonesia. The EUA is an approval for the use of drugs during a public health emergency. The four drugs are Favipiravir, Remdesivir, Regdanvimab, and Molnupiravir. Then in the Informatorium Edition 3 published by the Food and Drug Supervisory Agency (BPOM), the names of the Covid-19 therapeutic drugs included in the antiviral group, including Favipiravir, Remdesivir, Molnupiravir, Proxalutamide, and Oseltamivir are listed, “said Togi.
Although data from BPOM shows that the pharmaceutical industry in Indonesia is in the preparation stage for producing Covid-19 antiviral drugs, and some of them are already actively producing these drugs, Togi reminded that no drug is completely safe. This is because every drug must induces side effects. Therefore, the public is expected to take advantage of the official BPOM website or the “Halo BPOM” application to obtain accurate information related to drugs, so as to avoid hoaxes.
Another resource person, Prof. Dr. apt. Maksum Radji, explained that most of the antiviral drugs being developed, such as Favipiravir and Remdesivir, have a mechanism of action that is targeted at the RdRp (RNA-dependent RNA polymerase) of the Covid-19 virus, so that it will inhibit the replication process of the virus. “In addition to antivirals, there are also herbal plants that have the potential to be the drug of choice for Covid-19 therapy. In a research collaboration by UI and IPB, it was stated that the class of compounds that have the potential to be supplements in handling Covid-19 are hesperidin, rhamnetin, kaempferol, quercetin, and myricetin contained in guava fruit, orange peel, and Moringa leaves,” said Prof. max.
In his presentation, he also explained about the Covid-19 therapy algorithm which contains the management of Covid-19 and its treatment steps. The public discussion organized by FPUI Student Executive Board aims to make the pharmaceutical industry in Indonesia more independent in producing Covid-19 antiviral drugs.
Collaboration between researchers is also needed to continue studying and developing potential materials that can be used as the forerunners of Covid-19 drugs, including herbal plants.
With the circulation of various types of Covid-19 drugs, the public is expected to be wiser in filtering information which can be done by reading the official BPOM website as an accurate source and authorized party in regulating drug and food regulations in Indonesia.
Present at the event the Dean of the Faculty of Pharmacy UI, Prof. Dr. apt. Arry Yanuar, M.Si; apt. Dra. Togi Junice Hutadjulu, MHA. as Director of Production Supervision of Drugs, Narcotics, Psychotropics, and Precursors of the POM Agency, apt. Dra. Togi Junice Hutadjulu; and Professor of Pharmacy FIKES UEU and Retired Professor of FF UI, Prof. Dr. apt. Maksum Radji, M.Biomed. The event, which was held on Saturday (21/6) at 13.10 Western Indonesia Time, was held online via zoom and can be accessed in general on the YouTube channel of the UI Faculty of Pharmacy Student Executive Board.



